Archive
14. March 2022
Welcome to the team

We are pleased to announce that Harald Züger has joined the Effectum team. He is taking care of our financials and bringing them to the next level. Harald is a very skilled finance executive with 20+ years of comprehensive international, management and board experience. Since 2010 he has been providing finance and operational support for life science and healthcare start-ups in Switzerland, France and Austria and has worked in set-ups like Effectum before.
For more information about Harald visit his LinkedIn profile.
31. January 2022
5 year anniversary Effectum Medical
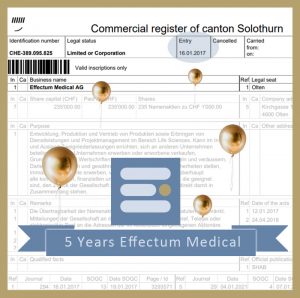
5 years ago, on January 16th, 2017 Effectum Medical was officially born. A big thank you to our great customer, partners and team without whom this journey would not be possible!
(more…)
09. December 2021
Effectum Medical joins the KAPSLY service-for-equity platform

Supporting start-ups is in our DNA. Therefore, we are very happy to announce that we have joined the KAPSLY platform! KAPSLY is a service-for-equity platform that connects service providers with promising start-ups.
We are the right partner to validate product concepts through the lens of regulatory requirements. Through our sister company MEDICALBOARD we can also offer Marketing and Strategy expertise and are thereby able to support start-ups throughout their product journey.
For more information about KAPSLY visit their website or get in touch!
29. November 2021
Effectum Medical & Medicalboard meets the slovenian business delegation

We are always happy to exchange ideas with opinion leaders from other countries, especially with countries that are extremely dynamic and have a high level of innovation.
Here are our 3 key take-aways in terms of regulatory environment: (more…)
08. November 2021
Interview with Nila-Pia Rähle

The Wirtschaftsförderung Olten has published an interview with Nila-Pia Rähle and introduced Effectum Medical AG to the region of Olten. Thank you very much for the opportunity to bring Effectum Medical closer to Olten.
You can find the whole interview here:
Newsletter Wirtschaftsförderung Region Olten
30. September 2021
FDA database for AI/ML-enabled medical devices

Artificial intelligence (AI), and specifically machine learning (ML), has become an important part of an increasing number of medical devices. Due to the increased interest in these medical devices, the FDA has basis a publicly available information created a list of AI/ML-enabled medical devices currently being marketed in the US.
The list is not an exhaustive or comprehensive resource of AI/ML-enabled medical devices but gives an overview of what is currently available.
Link FDA list
30. September 2021
In a row with M&A specialists and MedTech country leads

We feel honoured that Verve Ventures has interviewed Karina to receive insights about market access and regulatory. Read the full interview here.
21. September 2021
Paving the way for innovative medical devices – overcoming regulatory hurdles for development in academia

Not only a great contribution by DKF (Department of Clinical Research, University of Basel) and DBE (Department of Biomedical Engineering | University of Basel), but above all a pragmatic way how to succeed in the university sector
- – to quickly make innovative developments available to research
- – to keep the well-being of patients in mind.
We are honored to have been selected as a partner and to be able to assist with our quality management system and regulatory affairs expertise.
These are projects that fit Effectum Medical’s DNA – bringing medical device innovations to market efficiently and cost-effectively. Many thanks to Prof. Philippe Claude Cattin, Robin Sandkühler, Roland John, Christiane Pauli-Magnus, Dr. Unfer-Grauwiler Sandra for the great collaboration!
We are looking forward to the first market approvals and wish the team continued success!
For all interested parties, here is the access to the whole article, page 12-16
DKForum_Ausgabe17_Online-2.pdf (438 downloads )
02. September 2021
Why do we need a Swiss Authorized Representative (Swiss AR)?
Health Tech Cluster Switzerland’s event “Why do we need a Swiss Authorized Representative (Swiss AR)?” last week showed that interest remains high.
Our three Key Take Aways:
- The swissmedic sheet clearly lays out the two fundamental levels: Physical product responsibility – manufacturer, importer, distributor; Formal and safety concerns – CH REP.
- The role of the importer is significantly enhanced in the logistics chain, as he ensures that only products that comply with the MepV are placed on the market.
- Contrary to all expectations, swissmedic does not specify the location of the “Person Responsible for Regulatory Compliance”. The decisive factor is that he or she is able to exercise the responsibility in accordance with the legal requirements
Many thanks again to Stefan Leuthold for inviting Ulrike Neuberger. It was a pleasure to organize the event together with Beni Hirt and Michael Maier!
If you have any further questions, please feel free to contact us!
15. June 2021
Welcome to the team

Join us in welcoming Sarina Flühler our new Junior Quality Manager!
Sarina is just finishing her Bachelor of Science in Medical Engineering. 👩🎓 We wish her a lot of success and are convinced that she will graduate with flying colors.
We are convinced that her calm and thoughtful manner and her fresh knowledge will be a great benefit to our team.
For more information about Sarina please visit her LinkedIn profile.










